Prescription Drugs Utilization Review May Go Overboard in Its Oversight
Chemist's shop Benefit Administration
What entities are responsible for administering the Medicaid drug benefit?
States may administer the Medicaid pharmacy benefit on their own or may contract out one or more functions to other parties. The administration of the pharmacy benefit has evolved over time to include delivery through managed care (MCOs) and more reliance on pharmacy do good managers (PBMs). In addition, drug utilization review (DUR) boards and chemist's shop and therapeutics (P&T) committees play an oversight and administrative function in Medicaid pharmacy benefits. State contracts with MCOs, PBMs, or other vendors may have important implications for Medicaid enrollees and providers who are sometimes challenged by the administrative processes they must navigate to access or be reimbursed for medically necessary drugs. MCO subcontracts with PBMs are also under increasing scrutiny equally more than states recognize a need for more stringent oversight and management of these subcontract arrangements.
Managed Care's Function in Administering Pharmacy Benefits
Managed intendance plans play a major office in administering Medicaid chemist's shop benefits, with a majority of states using comprehensive managed care arrangements that include prescription drugs as a covered benefit. As of July ane, 2019, 40 states had comprehensive, risk-based contracts with one or more managed intendance organisation (MCOs).1 States with MCOs may opt to "cleave in" the pharmacy benefit by including it as a covered do good and placing the MCO at risk for costs, "carve out" the pharmacy benefit by excluding it from the MCO contract and covering drugs in FFS, or carve in some drugs and carve out others. Changes made to the rebate programme by the Affordable Care Act (ACA) take encouraged more states to cleave in the pharmacy benefit, allowing states to increment budget predictability and go out the MCO accountable for drug costs.2 , iii States that include chemist's shop as an MCO-covered benefit may also build administrative and clinical pharmacy requirements into the MCO contract, every bit discussed in more than detail later in this report.
Carve-out vs. Carve-in of the Pharmacy Benefit
In general, carving the pharmacy benefit into the MCO do good remains the prevalent arroyo for managing the prescription drug program for MCO enrollees. Of the 39 MCO states that responded to this survey question, simply iv states – Missouri, Tennessee, Westward Virginia, and Wisconsinfour – reported that chemist's benefits were generally carved out (with possible exceptions) equally of July 1, 2019, while the remaining 35 states reported that chemist's benefits were by and large carved in (Figure 1).
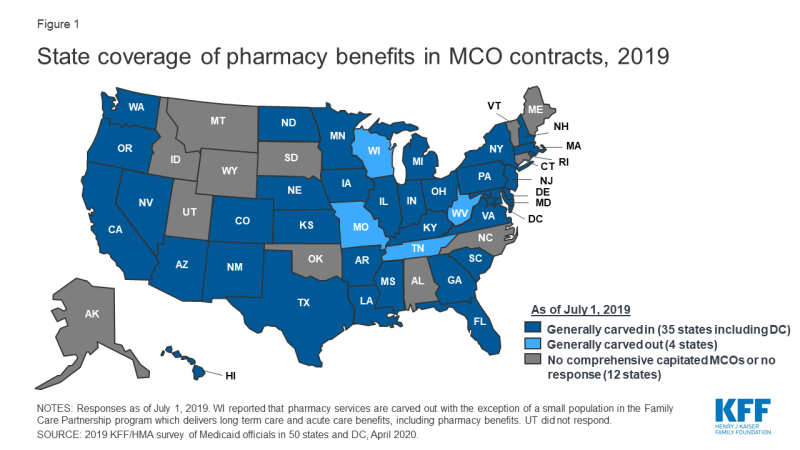
Effigy 1: Country coverage of pharmacy benefits in MCO contracts, 2019
Notwithstanding, more than states are making or considering carve-out changes. 2 states reported planned changes for 2020: North Dakota intends to cleave out the pharmacy benefit in 2020 and Wisconsin reported plans to cleave the pharmacy benefit out of its Family Care Partnership MCO contracts (serving a small number of delicate elderly and persons with disabilities) resulting in a full carve-out. Several other states reported that potential FY 2020 changes were "undetermined" or that pharmacy benefit commitment organization options were currently nether review. Illustrating the dynamic nature of this area, in the period since the survey was originally issued, California issued an RFP for a carve-out of its pharmacy benefit, to be constructive January i, 2021,5 and the Michigan Department of Wellness and Human Services announced its intent to cleave out the pharmacy benefit.half dozen
States are more likely to carve out certain subsets of drugs than to carve out the full benefit. Nosotros asked states nigh their utilize of carve-outs for certain high-cost drug groups/classes: 15 states report carving out one or more of these classes (Table 1 and Appendix Tabular array 1). Of those states, 12 (California, DC, Iowa, Indiana, Maryland, Michigan, Mississippi, New Hampshire, Ohio, Oregon, S Carolina, Washington) report using the class carve-outs as office of a risk mitigation strategy, as discussed in more item below.
| Tabular array 1 : Drug Classes Carved Out of MCO Benefit, July 1, 2019 | ||
| Drug Class | # of States | States (35 MCO Carve-in States Responding) |
| Hemophilia | nine | AZ, CA, FL, IN, MI, MS, NH, NJ, WA |
| Hepatitis C | 4 | IN, MI, SC, WA |
| HIV/AIDS | four | CA, DC, Md, MI |
| Mental Health | 4 | CA, Doc, MI, OR |
| Medication Assisted Therapy | three | CA, MD, MI |
| Oncology | i | MI* |
| Other | eight | AZ, IA, IN, Physician, NH, OH, TX, WA |
| NOTES: *MI reported information technology carves out select oncology drugs but not the unabridged class. SOURCE: 2019 KFF and Health Management Assembly (HMA) survey of Medicaid officials in 50 states and DC, Apr 2020. | ||
States are planning additional drug carve-outs for specialty or "blockbuster" drugs. For FY 2020, three states (Northward Dakota, Nevada, and Washington) reported plans to carve out additional drugs (with North Dakota intending to carve out the total chemist's shop benefit), and Maryland reported plans to implement both cleave-in and carve-out strategies. The nigh commonly mentioned drugs carved out or considered for cleave-out strategies are Zolgensma and Spinraza, factor therapy treatments for spinal muscular atrophy. Other agents mentioned include CAR-T therapies,7 cystic fibrosis, and muscular dystrophy drugs. Washington indicated it will accept a drug-specific approach to new carve-outs, reviewing specifically for impacts to the actuarial soundness of rates or for the likelihood that the fiscal impact of the new drug volition not be spread equally across Medicaid plans. While carving specialty drugs out of the pharmacy benefit is a more than common strategy, two states reported an intention to carve drugs back into the managed intendance do good in FY 2020: Maryland will carve in HIV/AIDS drugs and Michigan will cleave in hemophilia and oncology drugs and select drugs used to treat rare metabolic diseases.
Risk Mitigation Strategies
States that use MCOs to evangelize some or all chemist's benefits to Medicaid enrollees may use risk mitigation strategies to curb plans' financial risk. As of July 1, 2019, states reported deploying a multifariousness of fiscal risk mitigation strategies in their MCO contracts, with drug carve-outs and take chances corridors beingness the most common (Table 2). For drug carve-outs, hepatitis C and hemophilia drugs were those virtually normally mentioned among states with take chances mitigation strategies in place. Six states reported planned changes for FY 2020: Maryland, Nevada, and Washington are carving out additional drugs; Kansas is implementing different reimbursement methods for loftier-cost drugs; Ohio is removing the hepatitis C risk pool in 20208; and Michigan reported a plan to implement loftier-cost drug kicking payments for sure drugs being carved in every bit of October ane, 2019.
| Table ii: Risk Mitigation Strategies used in MCO Chemist's shop Contracts, July ane, 2019 | ||
| Strategy | # of States | States (35 MCO Carve-in States Responding) |
| Drug Carve-outs | 12 | CA, DC, IA, IN, Doc, MI, MS, NH, OH, OR, SC, WA |
| Risk Corridors | 10 | AZ, Hullo, KY, LA, MA, NE, NJ, NV, OR, RI |
| Kicking Payments | 4 | CA, KS, MD, MI |
| Hazard Pools | iv | DE, FL, NE, OH |
| Reinsurance | 3 | AZ, NV, VA |
| Other | 2 | MA, PA |
| None | eight | AR, GA, IL, MN, ND, NM, NY, TX |
| SOURCE: 2019 KFF and Health Management Assembly (HMA) survey of Medicaid officials in fifty states and DC, April 2020. | ||
The Role of PBMs and Other Vendors in Administering Chemist's Benefits
Country employ of external vendors to administer the pharmacy benefit, particularly the apply of PBMs, has generated considerable policy debate about costs and prices in Medicaid. While the relationship betwixt state Medicaid programs and PBMs is not new, the extent to which states rely on PBMs has grown significantly in the past ten years. PBMs may perform a variety of fiscal and clinical services for Medicaid programs, including adjudicating claims, administering rebates, monitoring utilization, supporting DUR processes, and overseeing and formulating preferred drug lists (PDLs).9 States may utilize PBMs in both managed care and FFS settings. The financial responsibilities PBMs take on, including negotiating prescription drug rebates with manufacturers and dispensing fees with pharmacies, have led to closer scrutiny of these arrangements and changing state policies governing PBM functions.
Fee-for-Service (FFS) Vendors
Inside FFS delivery of chemist's benefits, states accept historically outsourced some or all of the authoritative functions of the benefit and proceed to do so. All responding states except i (California) reported outsourcing some or all FFS functions to one or more than PBMs or other vendors as of July 1, 2019.x The nearly usually outsourced functions reported were claims payment and utilization management (Effigy 2 and Appendix Table 2). Other outsourced functions mentioned by more i state included provider call centers (Arkansas, Oklahoma, South Dakota, and Vermont) and determining AAC11 or MAC12 pricing (Indiana, Michigan, and Montana).
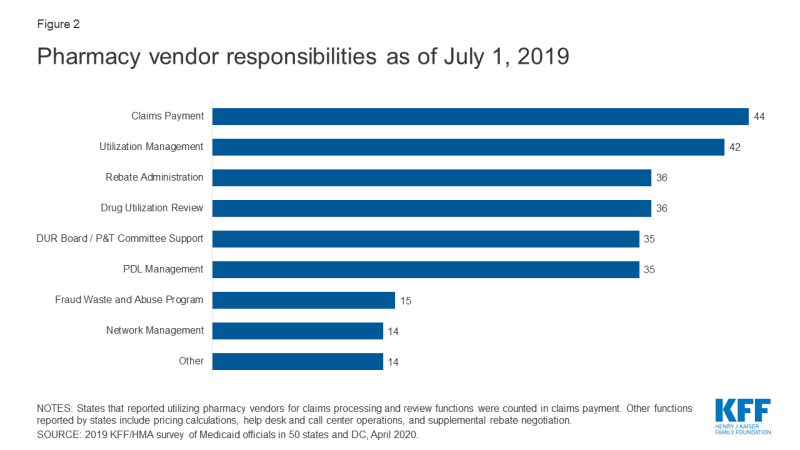
Figure ii: Pharmacy vendor responsibilities equally of July ane, 2019
In recent years, a number of states take consolidated pharmacy administration into more than streamlined contracts with a single vendor. These contracts resemble PBM contracts where the vendor has direct management responsibilities over the network, reimbursement rates, and rebate negotiations.xiii Of the 44 states that reported outsourcing the claims payment office, 23 reported that their fiscal intermediary, a vendor who handles authoritative functions on behalf of the country, processes chemist's shop claims14 with the remaining 21 respondents reporting outsourcing to Magellan, Change Healthcare, or OptumRx to process claims.
MCO Vendors and State requirements
States are taking activity to foreclose or monitor spread pricing within MCO-PBM contracts. 30-iv MCO carve-in states15 responded to survey questions nigh spread pricing. When the payment the PBM receives from the MCO and the reimbursement amount it pays to the pharmacy differ, some PBMs have opted to keep this "spread" as profit, an result which has generated a significant amount of political attention to MCO oversight.sixteen Eleven states prohibited spread pricing as of July 1, 2019; 5 states (Arkansas, Kentucky, Nebraska, New York and Washington) reported plans to eliminate spread pricing in FY 2020; and Maryland indicated that a spread pricing prohibition would accept effect in January 2021 (Figure 3 and Appendix Table 3). Also, Pennsylvania reported plans to require MCOs to develop a procedure that ensures pharmacy reimbursement is sufficient to cover acquisition costs plus the toll of professional services and the cost to manipulate. Twenty-half dozen MCO states reported they volition have transparency reporting requirements in place in FY 2020, including xvi states that crave MCOs to provide reports to the Medicaid agency.
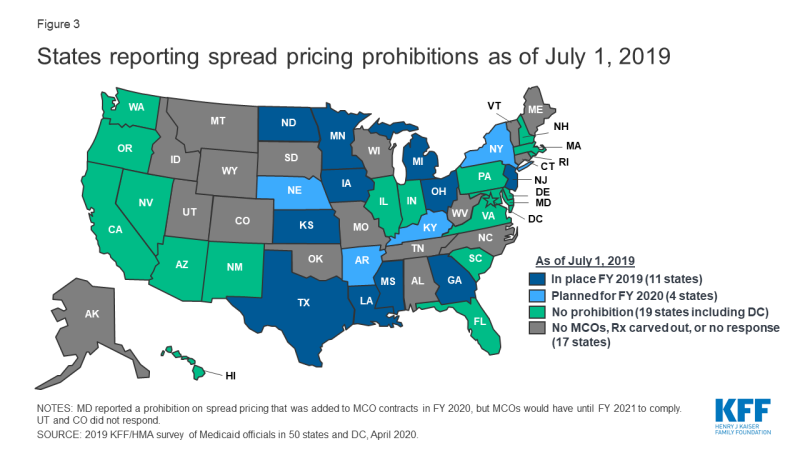
Figure 3: States reporting spread pricing prohibitions as of July 1, 2019
Outside of contractual prohibitions on spread pricing, very few states reported imposing other restrictions or limitations on the MCO relationship or contract with their PBM. States noted the following alternative deportment:
- In Delaware, two legislative job forces are reviewing PBM sub-contracting practices.
- Indiana is considering the level of reporting to exist required in 2021. While non contractually required, Indiana indicates that all MCOs accept moved to laissez passer-through PBM pricing.
- Kentucky'south MCO contract language includes an almanac PDL review, uniform alignment at the discretion of the agency, and mandatory laissez passer-through models; prohibits retroactive clawbacks of payments to pharmacies or imposition of fees, and removes contractual barriers to receiving claim or summary level information from the MCOs.
- Michigan reported plans to impose additional disclosure requirements as of October ane, 2019.
- Nebraska MCOs must obtain country approval of their pick of PBM
- On Jan 1, 2020, Washington volition prohibit retroactive payment adjustments (except by the MCO or in the upshot of an improper payment).
Drug Utilization Review Board (DUR Board) and Pharmacy and Therapeutics (P&T) Committee Policies
External boards and committees also play a part in overseeing and administering the Medicaid chemist's shop benefit. Section 1927 of the Social Security Act requires states to operate a DUR Board to guide drug utilization review (DUR) activities including retrospective review, awarding of DUR standards, and pharmacy and prescriber interventions. DUR programs also include evaluation for problems similar duplicate prescriptions, incorrect dosage, and clinical misuse. Data reported to CMS shows that 48 states take conducted a review of estimated cost savings or toll avoidance of their DUR plan, and CMS estimates that DUR programs salve about twenty%.17 States may also institute a P&T Commission to review therapeutic drug classes for PDL placement and coverage decisions, and 39 of the 50 responding states reported doing so every bit of July one, 2019.18 Arizona, which operates its Medicaid plan nether a Department 1115 waiver, reported having but a P&T committee, simply no DUR Lath. MCOs may besides use their own DUR Board rather than apply the same board as the FFS agency. Federal data for federal fiscal year 2018 shows that 28 states of 36 states with pharmacy benefits carved in (including Utah which did non participate in this survey) report that at least one of their MCOs has its ain DUR Board.19
Disharmonize-of-interest policies for DUR Boards and P&T committees are left to the states, and 10 states reported non having such a policy in place for at least one of these entities. Federal requirements for P&T committees specify they must consist of pharmacists, physicians and other "appropriate" individuals, merely otherwise leave states with flexibility for determining committee operations. Of the 50 responding states, xl reported having a disharmonize of interest policy for the DUR Lath.20 Of the 39 states that reported having a P&T committee, 36 had in place a conflict of involvement policy. Three states had no disharmonize-of-interest policy for either entity (Kentucky, Missouri, North Carolina).21
States vary in what functions DUR Boards and P&T committees play in administering Medicaid chemist's benefits. DUR Boards and P&T committees may play a role in setting or reviewing utilization management tools. With the exception of review of new drugs for PDL placement which was carried out by a P&T committee in more than half u.s.a., other activities were more than evenly divided across the various entities (Table three and Appendix Table iv). Most states that reported "other" entities were responsible for one or more of the activities commented that more than one entity was responsible for reviews. Yet, Connecticut reported that step therapy criteria are developed in legislation, Louisiana reported that its DUR Board reviews prior authorization (PA) criteria adult by the University of Louisiana Monroe's Office of Outcomes Research and Evaluation, and New Hampshire indicated that an independent medical review contractor was responsible for reviewing orphan/expedited review drugs.
| Table iii: Responsible Entity for Reviewing New PDL Drugs, Footstep Therapy Criteria, PA Criteria and Orphan/FDA Expedited Review Drugs, July 1, 2019 (50 States Reporting) | ||||
| Entity | New PDL Drugs | Step Therapy Criteria | PA Criteria^ | Orphan/Expedited Review Drugs |
| DUR Board | half dozen | 14 | 15 | 12 |
| P&T Commission | 29 | 12 | 9 | 10 |
| Medicaid bureau | 7 | xiv | 16 | 17 |
| Other | 4 | v | 9 | eleven |
| Northward/A | iv* | 5+ | 0 | 0 |
| NOTES: *States that reported they had no PDL. +States that reported they had no pace therapy. ^HI did not respond. SOURCE: 2019 KFF and Health Management Associates (HMA) survey of Medicaid officials in 50 states and DC, April 2020. | ||||
Most states reported that DUR Boards and/or P&T committees incorporate comparative effectiveness studies in coverage decisions. Over ii-thirds of the responding states (35 states) report reviewing comparative effectiveness studies when determining coverage criteria. The near unremarkably cited studies come from the Institute for Clinical Economic Review (ICER) and the Drug Effectiveness Review Project (DERP). However, states also report reviewing other drug effectiveness studies and compendia to determine effectiveness and several states use local universities and their DUR Board/P&T back up vendor for research.
johnstonsheas1990.blogspot.com
Source: https://www.kff.org/report-section/how-state-medicaid-programs-are-managing-prescription-drug-costs-pharmacy-benefit-administration/
0 Response to "Prescription Drugs Utilization Review May Go Overboard in Its Oversight"
Postar um comentário